Philips Pad Recall
Philips M5071A (adult) and M5072A (infant/child) AED Pads
A problem has been identified that could pose a risk for patients or users.
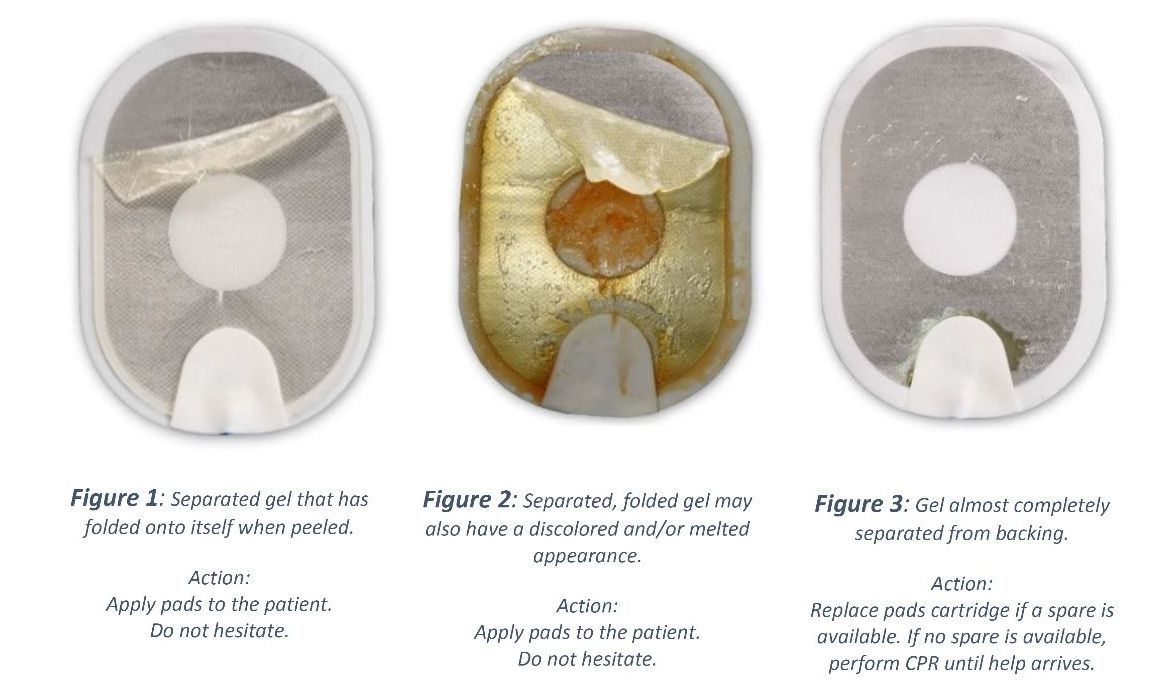
HS1/OnSite/Home AED pads (PN: M5071A, M5072A) have been observed to experience gel separation from the foam/tin backing when peeled from the yellow plastic liner. The gel may fold onto itself resulting in reduced surface area of gel on the pad, or it may separate almost completely leaving only a small amount of gel on the pad.
Any pad currently installed in or stored with an HS1/OnSite/Home AED could experience this problem, and it is not possible to know prior to patient use if your pad is affected because the pads are protected by a foil seal.
- A very small percentage of pads, approximately 0.0023 % have been identified as potentially affected.
- Over the past 10 years there have been 115 AEDs identified with the issue – with over 5 million AEDs sold in that time period.
We are here to help
Action First Aid is a Canadian National AED Distributor and can help answer any questions you have.
Email: info@actionfirstaid.ca
Toll Free: 1-866-347-7824